We may not need to cut down on our diet to live longer anymore. On 0.1-cm-long nematodes, scientists have found a molecular mechanism where dietary restriction promotes autophagy and delays aging. This finding has laid a clear path for determining the appropriate target of antiaging drugs.
Several animal models have proven the positive result of dietary or calorie intake restrictions in promoting autophagy and preventing senescence. Autophagy is an inherent function of the body cells that removes damaged organelles for cell regeneration. Moreover, it is more effective under certain conditions, such as controlling diet.
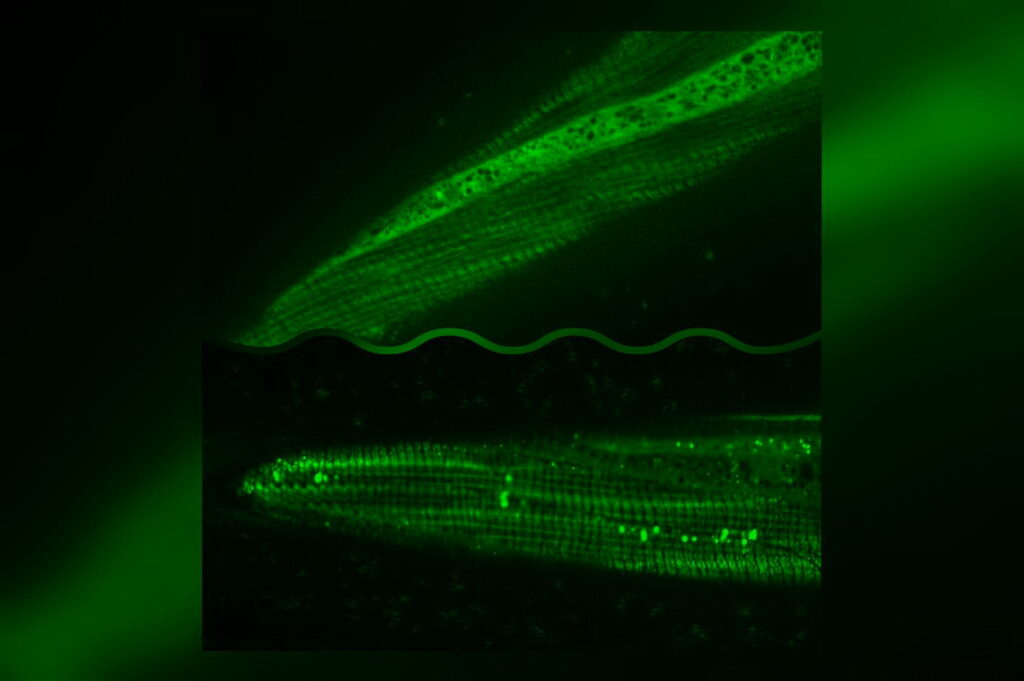
Autophagy activates cell self-regeneration and slows the aging rate
According to the latest research, scientists have found that nematodes extend their lives from 2 weeks on average to 3 to 4 weeks by eating a little bit less every day. Furthermore, they found the dietary restriction changed histonemethylation[1][2] in chromosomes through two critical proteases, SAMS-1 and SET-2, thereby regulating the activity of TFEB and FOXA, two transcription factors associated with autophagy, and improving the results of autophagy in nematode hepatic and intestinal cells.
When nematodes were fed less Escherichia coli, the result of SAMS-1 was low, which affected the histones’ methylation, allowing genes downstream of autophagy to be transcribed. Conversely, nematodes with a regular diet had a typical outcome of SAMS-1 and showed limited autophagy.
Nematode reveals ways to anti-aging without dieting
Prof. Ao-Lin Hsu of the Institute of Biochemistry and Molecular Biology of National Yang Ming Chiao Tung University (NYCU) presided over the study. He explained that based on the prior knowledge of dietary restriction being an effective way to delay aging, this finding revealed the molecular mechanism that underlies the relationship between dietary restraint and autophagy. He also said it showed the critical proteins that affect aging in dieting could help scientists determine the appropriate target of antiaging drugs and further develop aging-delaying methods that do not require dieting.
The aging rate can be affected by environmental factors and is an inevitable part of our lives. Dr. Hsu said that an appropriate practice of dietary restriction promotes cellular repair; under a condition with insufficient resources (e.g., dietary restriction), cells prioritize repair over reproduction regarding the use of resources for survival.
Why Nematodes?
In Hsu’s laboratory, nematodes are the primary animal used for testing; due to their biological similarities, the nematode is considered the first choice for aging research. They are nonparasitic worms that live in the soil. Compared with the longer life cycle of mammals such as mice, their shorter life cycle can reproduce in large numbers, and their essential genes are almost the same as humans.
Important harvest of cross-regions academic collaboration
This research is a joint effort between NYCU and Sanford Burnham Prebys Medical Discovery Institute. The research team includes Tsui-Ting Ching, an associate professor at NYCU Institute of Biopharmaceutical Sciences, and Chiao-Yin Lim, the first author and a PhD student at NYCU Institute of Biochemistry and Molecular Biology. The research is published in Autophagy, an international journal.
[1] A histone is a protein that provides structural support for a chromosome.
[2] Methylation is a biochemical reaction. The methylation of proteins inhibits or affects gene expression and is the foundation of epigenetics.

