As the Medical Devices Act opens access for universities to apply for medical device permits, NYCU has received official business permits from the Ministry of Health and Welfare for two medical devices developed in-house: the Isokinetic Testing and Assessment System and the AI Brain Tumor Detection System. This achievement is expected to significantly reduce the cost of clinical treatment.
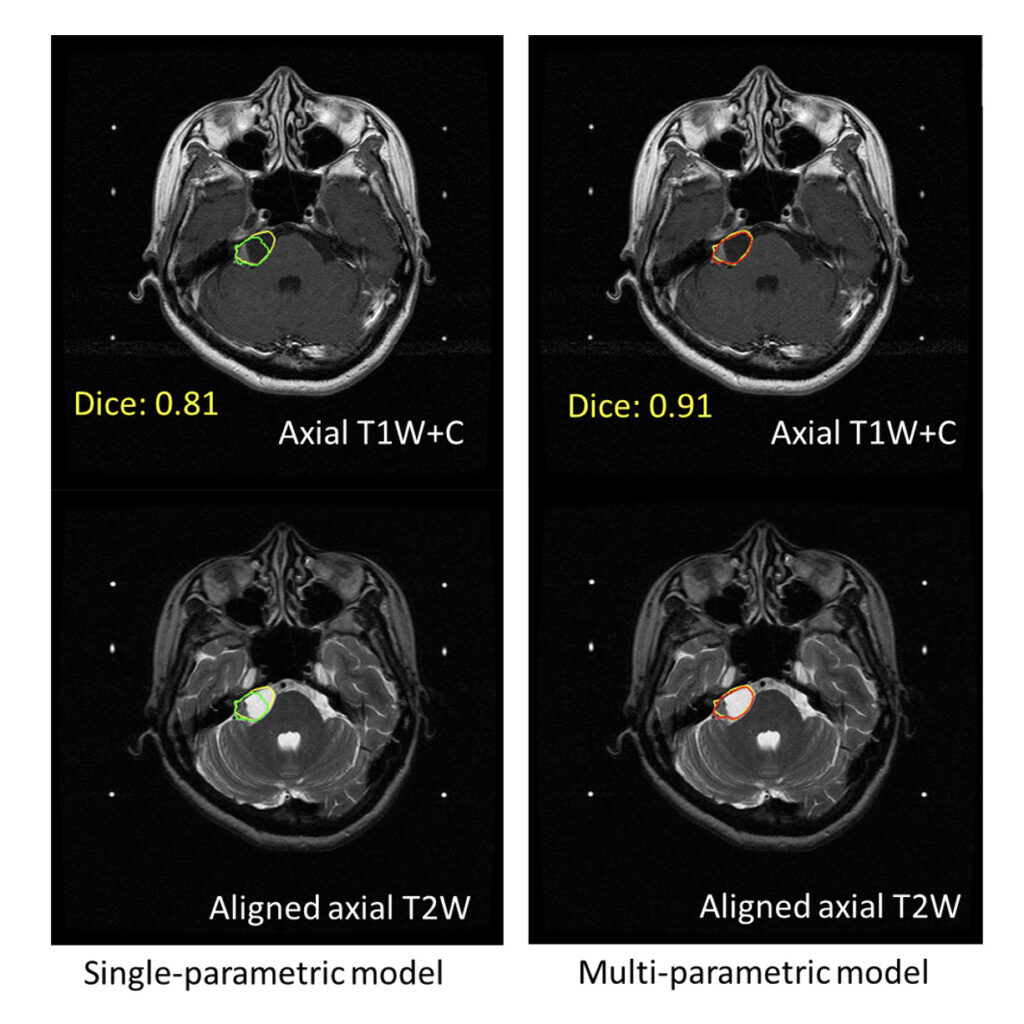
In the past, physicians relied on manual analysis of medical images to identify brain tumors. With the assistance of AI, brain tumor detection system can automatically analyze and mark magnetic resonance imaging (MRI) scans for three major brain cancers: acoustic neuroma, meningioma, and brain metastasis. This is the first intelligent medical software in Taiwan that is capable of automatic detection and marking of various brain tumors.
Professor Yu-Te Wu from the Institute of Biophotonics in NYCU, who is an expert in the field of AI brain tumor detection technology, stated that before radiotherapy, doctors need to review a large amount of image data to assess tumor volume, location, edema, and other pathological features. By leveraging AI learning from over 200,000 two-dimensional images from Taipei Veterans General Hospital and Taichung Veterans General Hospital, we were able to overcome challenges such as different axial and planar resolutions in clinical MRI images and the varying tumor types, sizes, and positions, enabling AI to accurately interpret brain tumors.
On the other hand, the isokinetic testing and assessment system for lip muscle strength in infants and stroke-affected elderly individuals addresses the clinical need for effective lip muscle rehabilitation and training.
Regarding the approval of these two medical devices by the Ministry of Health and Welfare, Professor Chun-Li Lin, Director of NYCU’s Medical Device Innovation and Translation Center, stated that universities are often at the forefront of medical device design and development. However, in the past, only companies could apply for inspection and registration of medical devices, which often delayed the marketization of medical innovations. Aiming towards clinical application, universities can now operate as medical device firms in addition to doing research and development as regulations become more lenient. This will facilitate the commercialization of additional research outputs.

NYCU Obtains QMS Certification and Business Permits for Medical Devices
In fact, the Medical Device Innovation and Translation Center has just received permits for its 3D-printed mandibular plate technology in April 2023, through the Quality Management System (QMS) of the Food and Drug Administration. NYCU has become the first university to possess both the QMS and first- and second-class medical device permits.
Chun-Li Lin stated that to enhance the quality of medical device research and development at the university, create value for the private sector, and facilitate technology transfer opportunities, NYCU will initially focus on medical software and assistive medical devices. Through a comprehensive service integrating clinical verification, regulation compliance, quality assurance, and commercialization, the university aims to accelerate medical device development, establish various product QMS, and achieve inspection and registration, ultimately promoting the commercialization of more research outcomes and advancing toward the goal of clinical application.
