▲ Led by Associate Professors Tsai-Wen Chen and Bei-Jung Lin at NYCU, a groundbreaking study using advanced imaging revealed that inhibitory interneurons in the hippocampus form selective “cell assemblies,” reshaping understanding of memory formation and offering new directions for treating neurological diseases ©NYCU ELITE
The hippocampus is an important brain structure that affects spatial and directional perception, and closely relates to forming and consolidating memories. Abnormal functioning or damage to it can lead to various diseases such as Alzheimer’s disease, epilepsy, depression, and Schizophrenia. Two associate professors, Tsai-Wen Chen and Bei-Jung Lin, from the Institute of Neuroscience of National Yang Ming Chiao Tung University (NYCU), recently conducted research that reveals the relatively rare neurons in the hippocampus—the “interneurons”—have a special “cell assembly” behavior, which not only subverts the previous understanding of the mechanism of the hippocampus but also brings a brand-new inspiration for the treatment of related brain disorders. The results of this research have been published in Neuron, a leading international journal, marking a significant milestone in neuroscience.
Inhibitory interneurons, a few but crucial players
Professor Lin stated that about 90% of the neurons in the hippocampus are excitatory projection neurons, while the remaining 10% are inhibitory interneurons. When external information enters the hippocampus, these two types of neurons collaborate in processing it, and the excitatory neurons subsequently send the result to the downstream brain areas. Professor Chen explains that the functions of these two types of neurons are like the gas pedal and a car’s brake—if you only step on the gas pedal but not the brake, the car will easily go out of control. Similarly, if the excitement signals in the brain are too strong and there is not enough inhibition to counterbalance them, abnormal discharges like epilepsy may occur, highlighting the importance of the inhibitory neurons.
In addition, inhibitory interneurons play an essential role in producing brain waves, especially “ripple brain waves,” which are highly related to memory formation and consolidation. Professor Lin cited the example of mice performing a maze-like task. If the mice’s ripple brainwaves are damaged, their memory of the maze route is impaired. The neurotransmitters secreted by the inhibitory interneurons are also related to maintaining regular sleep. Some over-the-counter sleeping pills are designed to enhance the release of these neurotransmitters. “Diseases such as Schizophrenia and depression are often linked to the weakening or loss of functions in interneurons. Understanding how interneurons work will help develop better therapeutic drugs,” Professor Lin emphasized. Despite their small size, the functions of the interneurons are extremely important, making them a popular research topic in contemporary neuroscience.
Breaking the technological bottleneck, no more needle in the haystack
Neurons generate electrical signals, known as action potentials, through rapid potential changes inside and outside the cell membrane, which are then transmitted to the next neuron via synapses. The entire neurotransmission process is speedy, typically taking only a few milliseconds. Professor Chen pointed out that in past research, electrical signals were usually recorded by inserting electrodes thinner than a hair into the neurons. However, this approach made it difficult to pinpoint specific cells, and most could only be measured by “blind insertion,” namely, randomly inserting it into a cell. Moreover, there are not only a small number of interneurons but also more than two dozen different types of interneurons, making the likelihood of successfully targeting a specific cell extremely low. Pinpointing a particular kind of interneuron for the study was akin to “searching for a specific small mushroom in the mountains.”
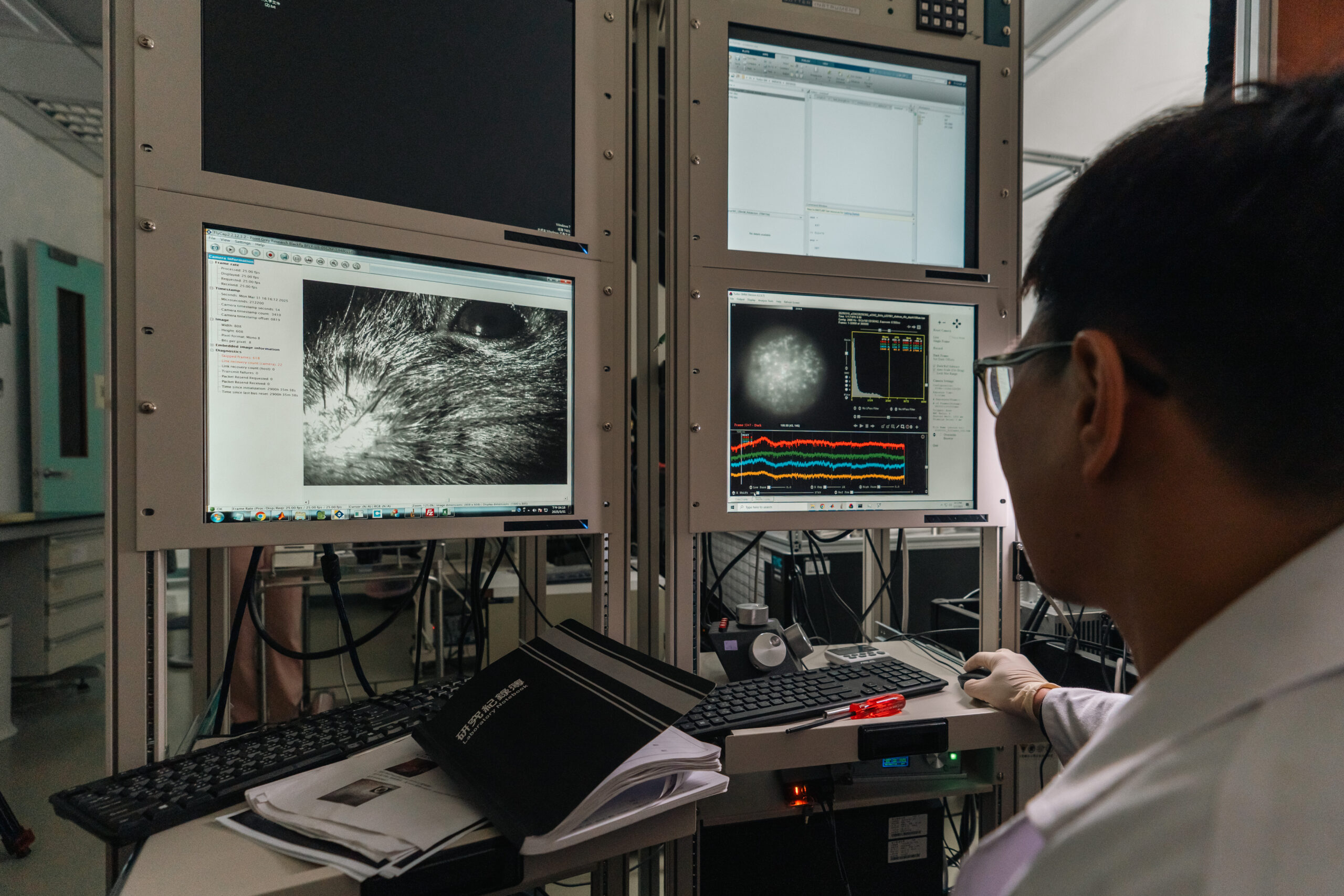
Professor Lin said that the primary focus of this research is the PV-positive interneuron, which has the fastest and highest discharge frequency among neurons. Due to their scarcity, only sporadic single-cell records were available in the past, and it was impossible to understand the synchronization and communication between cells. To break through this limitation, the research team employed an advanced imaging technique to implant a new generation of “voltage-sensitive fluorescent proteins” into the brains of mice by viral injection. When PV cells produce membrane potential changes, the brightness of the cellular fluorescence will change according to the level of the potential. To record the action potential signals, the research team set up an imaging system that can take more than 2,000 photos per second, achieving the world record of recording up to 26 PV cells simultaneously.
Professor Lin added that the intracellular electrophysiology method allowed only one electrode to be inserted into a mouse at a time. At the end of the experiment, apart from the sacrifice of the mouse, the right target cells might not be found. The new method is to surgically add a transparent experimental window on the mouse’s skull, dramatically increasing the number of PV cells that can be successfully observed and allowing the same mouse to be used for multiple experiments. This technique is a concrete implementation of the 3R principle—Replacement, Reduction, Refinement—in experimental animals.

Cells also make friends with a variety of making-friend patterns
Professor Lin pointed out that in the past when scientists studied the phenomenon of “cell assembly,” where neurons work together as a group (e.g., synchronized discharge), they largely believed it occurred only in excitatory projection neurons. However, the research team proved for the first time that the “cell assembly” phenomenon also occurs in intermediate neurons.
“We were surprised to learn that it was previously believed all interneurons operated in synchronization within the network. Nonetheless, they are actually divided into groups, with each cell having its own ‘best friend.’ This group of cells will activate and act together, not just randomly pull a cell to work together. Professor Chen further cited ripple brain waves as an example, explaining that each cycle of ripple brain waves lasts only 6 or 7 milliseconds. During this brief period, interneurons connect with different “friends” to activate together with each cycle. This shows the diverse behavior of interneurons in “cell assemblies,” suggesting they may have a more active role in memory formation and consolidation.
As to how interneurons find their “best friends,” the research team suggested that there may be some strong “connection” between these cells, such as relying on the release and connection of neurotransmitters or connecting by “Gap Junction” to form a synchronized discharge network. Professor Chen mentioned that while current imaging techniques cannot directly observe these physical connections made by gap junctions, this area is one of the topics worth studying in the future.
All-optical + two-way communication design to deepen the understanding of the “cell assembly” behavior
“Voltage-sensitive fluorescent protein” and “ultrafast photography” have brought breakthroughs in neuroscience research. Looking ahead, the research team aims to apply these two technologies further to study excitatory projection neurons. Professor Lin pointed out that a large number of excitatory projection neurons and their overlapping features may cause the images of these neurons to appear blurred when labeled with fluorescent proteins. The research team is focused on developing a new generation of imaging solutions, and the relevant results will be published in international journals recently. In addition, the research team will further investigate the interaction between excitatory projection neurons and interneurons, especially by examining their roles and functions in brainwave formation.
The team is also actively developing “all-optical electrophysiology” technology incorporating “voltage-sensitive fluorescent proteins.” Their goal is to utilize light as a replacement for electrodes to stimulate neurons and record electrical signals through changes in cellular fluorescence. Professor Chen said that besides unidirectional reading and recording electrical signals from neurons, the team is also very interested in “inputting information” to measure cell connections. In the future, they will conduct the research in the direction of all-optical, two-way communication. The research team expects that by deepening the understanding of the behavior of neuronal grouping, more precise techniques for diagnosing and treating neurological diseases, as well as regulating brain function. This advancement is expected to be developed to move towards a new era of brain science.

Interview | Fu-Kuo Chu
Translation | Yi-Chen Emily Li
Editing | Hsiu-Cheng Faina Chang
Photography | Hao-Yun Peng and Zong-Han Lyu / ZDunemployed Studio
