▲ NYCU Assistant Professor Sung-Fu Hung and his international team have developed a world-first carbon-negative technology that efficiently converts CO₂ into methane using triazole organic molecules, marking a major breakthrough published in Nature Energy ©NYCU ELITE
According to a study report by the United Nations, global greenhouse gas emissions reached 57.1 billion tons of carbon dioxide equivalent (CO2e) in 2023. This marked a 1.3% increase compared to 2022, reaching an all-time high. According to the Global Carbon Project, only the global CO2 emissions from fossil fuels grew by 0.8% in 2024 compared to 2023 to reach 37.4 billion tons, and the annual carbon emissions were also not promising. These figures have been a wake-up call for the global climate crisis and prompted scientists to look for breakthrough solutions to reduce carbon emissions.

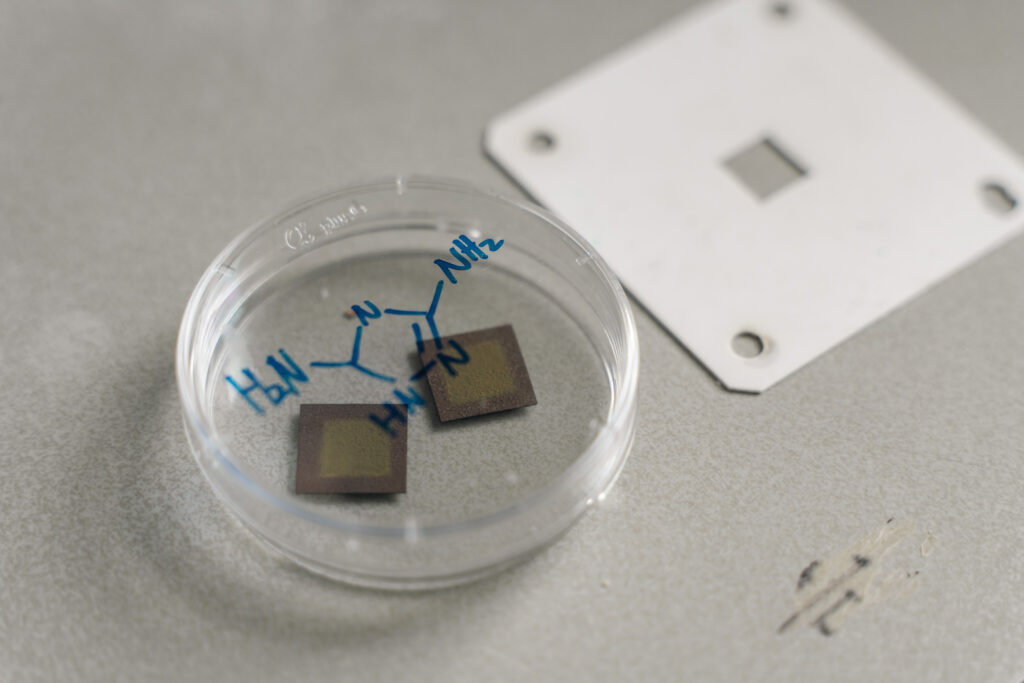
Sung-Fu Hung, the Assistant Professor at the Department of Applied Chemistry of National Yang Ming Chiao Tung University, has collaborated with Ying Wang, the Assistant Professor at The Chinese University of Hong Kong, and Ziyun Wang, the Senior Lecturer at the University of Auckland, to comprise an international research team. In the electrocatalytic CO2 reduction reaction, the innovative use of “triazole organic small molecules” as catalysts has successfully and highly efficiently converted CO2 into methane, an essential component of natural gas that can be directly used as a fuel in daily life.
This world-leading revolutionary technology perfectly realizes the sustainability goal of the carbon cycle and also brings new hope for global carbon reduction. The related research results were published in the leading journal Nature Energy under the title “Electroreduction of CO2 to methane with triazole molecular catalysts”, attracting significant attention from academia and industry.
Turning the Impossible into Possible
Professor Hung, who specializes in heterogenous catalysts, reactor design, and in-situ observation technologies, has focused to exploring the application of zero-carbon and negative-carbon technologies in recent years. Professor Hung said that zero-carbon technology focuses on reducing reliance on fossil fuels. Currently, the mainstream approach is to use hydrogen to replace conventional fuels, which can reduce CO2 emissions. However, it just prevents the emission of additional CO2, rather than actively reducing the existing atmospheric CO2 concentration needed to effectively mitigate the greenhouse effect and address climate change in a timely manner. In contrast, negative carbon technology, which converts carbon dioxide into small molecules (e.g., methane, ethanol, etc.) that can be utilized to consume CO2 to ameliorate environmental hazards directly, is a key area of exploration for industry and academia at present.
The catalytic reduction of carbon dioxide is mainly divided into three mainstream technologies: thermal catalysis, photocatalysis, and electrocatalysis. Because thermal catalysis is energy-consuming and produces relatively low-end products, and photocatalysis yields low production with low yields, Professor Hung’s team focuses on and is devoted to electrocatalytic technology, which has a high conversion rate and a wide range of products. Currently, most electrocatalysts adopt relatively expensive metals such as copper and silver as catalysts. In contrast, the organic small molecule catalysts, which were not favored, unexpectedly attracted the research team’s attention at this time.
The research team found in the literature that nitrogen-containing small molecules have certain catalytic activity (i.e., the ability to promote the reaction) for the CO2 reduction reaction and theoretically confirmed that triazole molecules containing three nitrogen atoms have excellent catalytic performance. In particular, the amine group next to the triazole molecule is a nitrogen-containing basic functional group capable of efficiently adsorbing weakly acidic CO2. This functionality not only plays a critical role in initiating the entire conversion reaction, but also contributes to enhanced catalytic efficiency by promoting effective CO₂ activation and facilitating subsequent reaction steps. The team finally succeeded in reducing CO2 to methane after introducing water vapor (which provides hydrogen molecules).
Professor Hung explained that methane, as a higher-end product of CO2 reduction, is more difficult to generate than primary products such as carbon monoxide, and methane is the main component of natural gas. Therefore, this is the first artificial technology worldwide to realize a highly efficient CO2 electrocatalytic reduction reaction with triazole organic small molecules and recycle CO2 to generate methane, which is of landmark significance in replacing fossil fuels. Professor Hung proudly said, “This is the first research that has the potential for industrialization, making the ‘impossible possible.’”

Realizing Sustainable Carbon Cycle
In addition to the breakthrough in the catalyst, the research team also made significant progress in designing the electrocatalytic reactor. The conventional electrocatalytic reaction is carried out in an electrolysis cell, where CO2, an electrolyte, and an electrode are configured to facilitate the desired electrochemical processes. However, the solubility of CO2 in aqueous solution is only 5%, severely limiting the catalytic activity. Therefore, the team adopted two new types of reactors, a Membrane Electrode Assembly (MEA) and a flow reaction cell for improvement.
The research process involves directly feeding CO2 gas into one side of MEA and adding electrolytes to the other side, which solves the problem of low CO2 solubility on the one hand and increases the catalytic activity by 100 to 1000 times on the other hand. The research results show that the triazole organic small molecule catalyst can be stably operated in the MEA for 10 hours at a current of 10 amperes. The methane production rate reaches 23.0 millimoles (mmol) per hour, with a conversion rate (Faraday efficiency) of 52 ± 4%, relatively close to the industrialized mass production standard of 60%. In addition, by regulating the fed water vapor to carbon dioxide ratio, the plant can directly generate coal gas for human consumption, realizing a sustainable carbon cycle from CO2 to fuel.
It is worth mentioning that the area of MEA used by the research team has been expanded from 1 square centimeter, which is commonly used in general laboratories, to 80 square centimeters. In the future, if 10 MEAs are connected in series to form a stack module, the total output is expected to increase by 9 to 10 times, showing that this technology has the potential for industrialized application.
The success of this research is mostly due to the close collaboration of the international team. Professor Hung, Ying Wang and Ziyun Wang worked together at the University of Toronto in Canada. In this research, Professor Hung and Ying Wang were responsible for the catalysts, reactor planning and design, and in-situ observation of the catalytic reaction, while Ziyun Wang focused on the theoretical calculations and analysis of the reaction mechanism.
“In addition to demonstrating the activity of small molecules, exploring the reaction mechanism is also important. The team used in-situ observation and isotope analysis to successfully record the key data of the interaction between CO2 and the catalyst. Then, the data was submitted to researchers in New Zealand for computation, leading to a thorough analysis of the whole catalytic reaction mechanism. This research process of linking the experimental end and theoretical end is more time-consuming than expected,” Professor Hung added.
Accelerating Carbon Footprint Elimination
As the time schedule of net-zero emissions by 2050 is approaching, issues such as carbon tax and carbon footprint are getting more and more attention from all walks of life, especially the chemical industry, which often emits a lot of CO2 in its processes, is under pressure of high carbon tax. Even the semiconductor industry, which is praised as “Taiwan’s silicon Shield,” uses a lot of electricity, keeping the carbon footprint high. The novel carbon-negative technology led and developed by NYCU will substantially assist in solving the carbon footprint problem.
Professor Hung pointed out that the chemical raw materials generated by the new technology, such as methane, ethanol, or acetic acid, can be directly utilized or sold at a profit by enterprises, and the carbon cycle formed by converting carbon dioxide into methane can also reduce carbon footprint and carbon tax payments. However, at this stage, Professor Hung believes that there are still many challenges to be overcome before the new carbon-negative technology can be formally applied on an industrial scale, including how to deal with the various impurities in the gases emitted by different industries so as to avoid affecting the performance of the reactors and catalysts, as well as how to interface the reactors with the existing plant systems.
In the future, the research team will focus on improving the conversion rate and conversion capacity, aiming to increase the catalytic activity to more than 1000 times the current level, attracting more industries’ interest and accelerating the technology implementation. At the same time, the team will continue to optimize the reactor design to solve the problems of flow balance between raw materials and products so as to avoid device design problems affecting the catalyst activity. The research team also plans to explore the hybrid design of organic molecules and metal catalysts to achieve a higher conversion rate and product diversity in the future.
“Our ultimate vision is to bring CO2 emissions back to pre-industrial levels, so it is even more important to accelerate the development of carbon-negative technologies when global carbon emissions cannot be effectively reduced. This is the assistance that scientists can and must provide along the road to net-zero emissions and industrial transformation,” Professor Hung emphasized.
Apparently, a carbon reduction revolution that “turns stones into gold” is ready to be launched!

Interview |Fu-Kuo Chu
Translation | Yi-Chen Emily Li
Editing | Hsiu-Cheng Faina Chang / StoryLab
Photography | Hao-Yun Peng and Zong-Han Lyu / ZDunemployed Studio
